|
Chapter 5: Health Risk Assessment
The methodologies and techniques used in health risk assessment are firmly established. This chapter provides an overview of current health risk assessment methodologies used for physical, chemical and biological agents and reflects the typologies in Chapter 3. The influence of the NAS model can be seen clearly in chemical and biological risk assessment. Risks to human health from site-specific industrial activities are covered in Chapter 7.
5.1 Physical Risks - Ionising Radiation
Radiation risk assessment methodologies are well developed and, due to the nature of nuclear risks, many international organisations are involved. The book is not concerned with the scientific arguments surrounding the biological effects of radiation, only its use in radiation risk assessment. Assessments of risk are carried out by the regulatory agencies involved in radiological protection - setting radiological dose limits for instance and site-specific assessments, and by the nuclear industry - compliance with legislation and site-specific decisions. Radiation risk assessment has a longer history than that for other types of risk and the influence of the NAS model, developed for human health risk assessment for chemicals, is less marked. Of the many international bodies involved in radiological protection and radiation risk assessment, the International Commission on Radiological Protection (ICRP) and the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) are influential on a global scale. UNSCEAR does not recommend risk management action but carriesout evaluations of doses, effects and risks of radiation which are then used by bodies such as the ICRP. ICRP has had a major influence on European legislation on radiological protection through the EURATOM Directives.
The book describes the risk assessment methodology used by the ICRP. The hazard identification/dose-response steps are explained with reference to deterministic and stochastic effects and the biological effects of ionising radiation at low doses. Exposure assessment and risk estimation are described briefly. An example of a site-specific risk assessment is provided.
5.2 Chemical Risks
5.2.1 EC Legislation and Technical Guidance
The procedures, methods and techniques for regulatory risk assessment of chemicals in the EU is described in both legislation and supporting Technical Guidance Documents. Implementation is supported by the European Chemicals Bureau, part of the Joint Research Centre, in Ispra.
International Organisations such as OECD, IPCS and ECETOC and many National Organisations are conducting programmes on human health and ecological risk assessment. The work contributes both to the shaping of regulation and the response to regulation.
5.2.2 Human Health Risk Assessment for Chemicals
Most methodologies for human health risk assessment of chemicals are based on the NAS model. A number of methodologies exist due to differences in the toxic mechanisms exerted by different classes of chemical and the toxicological end-point being assessed. The end-point being assessed could be death, or a specific pathological condition relating to exposure to a chemical. When attempting to assess the risks from an immuno-suppressant toxin, specific end-points may be difficult to determine, as may be the role of other agents and stressors on the body. This will lead to risk assessment methodology for immuno-suppressants being different from assessments for irritants for instance.
All human health risk assessments of chemicals include hazard identification, dose-response assessment, exposure assessment and risk estimation/characterisation. If the assessment is site-specific, then a release assessment would be required in the absence of good data of environmental levels or to account for non-routine, accidental releases.
Hazard Identification is defined as "the identification of the adverse effects which a substance has an inherent capacity to cause" (CEC, 1993). This involves consultation of any toxicological and epidemiological data. Sources of such information can be found in the Information Sources Section 2 on databases.
Dose-response Assessment
Dose-response assessment is the "estimation of the relationship between dose, or level of exposure to a substance, and the incidence and severity of an effect" (CEC, 1993). The dose-response relationship is ascertained from epidemiological and toxicological data. See databases.
Exposure Assessment
Exposure assessment is described as the "determination of the emissions, pathways and rates of movement of a substance and its transformation and degradation in order to estimate the concentration/doses to which human populations or environmental compartments are or may be exposed" (CEC, 1993).
Environmental exposure to chemicals can be direct - as a result of emission to the environment (air, land, water) of a substance through industrial manufacture, use or disposal, or indirect - through drinking water or the food chain.
The possible routes of exposure to chemicals and the main factors to be considered are summarised in Figure 5.1
Figure 5.1. Estimation of Human Exposure Major Routes (source UK Government and Industry Working Group, 1993)
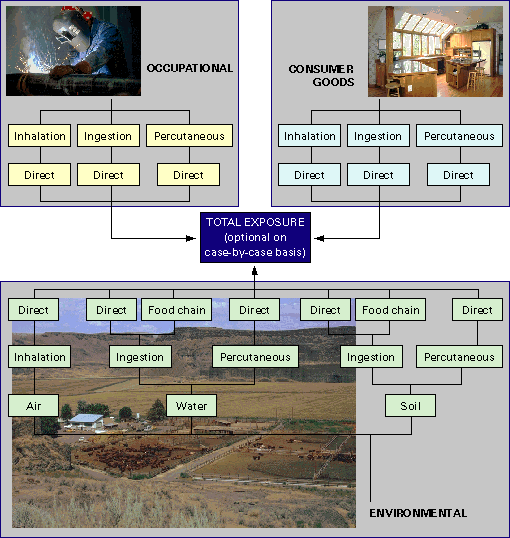
Exposures can be estimated from direct measurement of the chemical in the environment, or chemicals or metabolites in the body. They can also be predicted from the use of fate, transport and exposure modelling techniques.
Risk Characterisation
This is defined as "the estimation of the incidence and severity of the adverse effects likely to occur in a human population or environmental compartment due to actual or predicted exposure to a substance, and may include "risk estimation", i.e., the quantification of that likelihood" (CEC, 1993). Essentially, risk characterisation is a summary of the data compiled in the risk assessment process including the uncertainties associated with each stage and the presentation of a risk estimate. For new and existing chemicals it will lead to conclusions for further action.
The book describes the above four stages of health risk assessment in far more detail and then goes on to describe the following different typologies of health risk assessment as outlined in chapter 3, using examples to illustrate each:
- Neurotoxic (examples of lead and pesticides)
- Immunotoxic (example of allergenic substances)
- Developmental (example of thalidomide)
- Reproductive (example of phthalates)
- Carcinogenic (example of dioxins)
5.3 Biological Risks
Biological risks can be separated into those risks associated with biological agents of concern to public health such as pathogenic strains of bacteria, which are of particular concern as food-borne hazards, and the introduction of genetically engineered organisms into the environment or the food chain. The field of risk assessment of biological agents is relatively novel but, as with chemical risk assessment, it has become a major management tool. The World Health Organization has adopted risk assessment as the main way to scientifically justify food safety standards (FAO/WHO, 1995). However, significant problems exist when applying quantitative risk assessment techniques to microbial hazards, such as the difficulties in obtaining dose-response data and elaborating appropriate dose-response relationships in humans (Christiansen, 1996).
5.3.1 Food Safety Risk Assessment

Vanessa Miles, Environmental images
|
The responsibility for setting standards, recommendations and guidelines on food safety on an international scale rests with the Codex Alimentarius Commission (CAC), set up by the Joint Food and Agricultural Organisation of the United Nations (FAO) and the World Health Organization (WHO) Food Standards Programme. Biological agents (hazards) of concern to public health include pathogenic strains of bacteria, viruses, helminths, protozoa, algae, and certain toxic products that they may produce. Currently, the presence of pathogenic bacteria in foods presents the most significant problem internationally. The CAC looks at biological and chemical risk in food and have described the basis of their risk assessment methods (CAC, 1993, FAO/WHO, 1995).
|
It is derived from the NAS model and has four components involved in the assessment of biological agents which are:
- Hazard Identification
- Hazard Characterisation
- Exposure Assessment
- Risk Characterisation
Risk assessment is regarded by the CAC as an essential tool in its role of producing guidelines, standards and recommendations to protect human health from food-borne hazards.
5.3.2 Risk Assessment of Genetically Modified Organisms
The procedures for carrying out health and environmental risk assessments for the deliberate release and contained use of genetically modified organisms are set out in two EC Directives (CEC, 1990). This legislation provides a framework for EU States to build their own individual programmes. The methodology developed from the Directives in the UK for health and environmental risks is given in Chapter 6 although emphasis is placed on environmental risks. It is acknowledged that many uncertainties prevail in the risk assessment of GMOs due to the open interpretation of the EC Directives and the primitive stage of development of the methodology.
Reference list for this chapter
Many of the sections in the Information Sources are relevant to this chapter but particularly:
Databases (chemicals)
Software Models (exposure)
Publications (books)
|


Document Actions
Share with others