|
Chapter: 6 Ecological Risk Assessment

Leslie Garland, Environmental images
|
Ecological Risk Assessment (EcoRA) involves the assessment of the risks posed by the presence of substances released to the environment by man, in theory, on all living organisms in the variety of ecosystems which make up the environment. EcoRAs tend to focus on the risks from chemicals and Genetically Modified Organisms (GMOs), some address physical risks such as temperature rises caused by cooling water releases from industry.
|
EcoRA methodology has been developed from that already established for human health. The general principles are widely agreed upon but the application of the process still provokes considerable argument. The Health Risk Assessment (HRA) approach lends itself well in many respects to EcoRA but, due to the complex nature of the potential target(s) or receptor(s), several problems have presented themselves to practitioners. HRA is concerned with individuals and morbidity and mortality, EcoRA is concerned with populations and communities and the effects of substances on mortality and fecundity. EcoRA has to deal with a multitude of organisms, all with varying sensitivities to chemicals and various groups have distinct exposure scenarios, such as free swimmers and sediment dwellers. Because of the difficulty in obtaining toxicity data on all organisms in an ecosystem, the recognised practice is to test selected representatives of major taxonomic groups and use these as surrogates for the whole system. This method is questionable as it may not protect the most sensitive species exposed in the environment. Failure to identify the effects of an agent on a potential receptor can result in widespread damage to organisms and ecosystems. A typical example is the use of anti-fouling paints containing tributyltin and the resulting damaging effect on oysters and dog whelks.
6.1 The Risk Assessment Process for Chemicals
The book describes, in detail, the methodology for ecological risk assessment used in the regulation of new and existing substances in the EU, as described in the EU Technical Guidance Documents as environmental risk assessment.
The method consists of the four steps used in health risk assessment although Hazard Identification and Dose-response Assessment are combined in the single step Effects Assessment:
- Effects Assessment involves the identification of the hazard based on its physico-chemical properties, ecotoxicity and intended use, and the estimation of a Predicted No Effect Concentration (PNEC), derived from ecotoxicity data and the application of assessment factors;
- Exposure Assessment involves the calculation of a Predicted Environmental Concentration (PEC). This is derived using monitoring data, realistic worst cases scenarios and predictive modelling techniques. It is a complex task and should consider release, degradation, and transport and fate mechanisms. Local relevant emission and distribution routes are shown in Figure 6.3.
- Risk Characterisation involves the calculation of a quotient - the PEC/PNEC ratio. If the ratio is less than 1 the substance is considered to present no risk to the environment in a given scenario.
Similar schemes have been developed by the OECD and others.
|
Figure 6.3
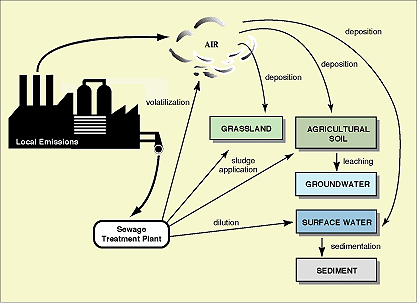
Local relevant emission and distribution routes (source: CEC/ECB Technical Guidance Documents in Support of the Commission Directive 93/67/EEC on Risk Assessment for New and Notified Substances and the Commission Regulation (EC) 1488/94 on Risk Assessment for Existing Substances. P 295. 1996. In press.)
|
Ecological risk assessment is very much a developing field and has many problems which need resolving such as;
- Determining the effects at population and community level;
- Selection of end-points;
- Selection of indicative species;
- The selection of field, laboratory, mesocosm and microcosm tests;
- The incorporation of resilience and recovery factors of the ecosystem.
The problems are covered comprehensively in such texts as Suter (1993) and van Leeuwen and Hermans (1995).
Many organisations are actively involved in the development of ecological risk assessment methodology, they include the US EPA, the US NRC, OECD, SETAC and ECETOC.
6.2 Ecological Risk Assessment of Plant Protection Products

Patrick Sutherland, Environmental images
|
Although classified, of course, as chemicals, pesticides have been considered as a separate category of substances requiring their own regulation due to their wide distribution and the nature of their use.
|
Many countries and international bodies have developed their own procedures and guidelines for the risk assessment of plant protection products. A joint panel of the European and Mediterranean Plant Protection Organisation and the Council of Europe have developed a scheme which aims to provide a framework for the evaluation of risks leading to the approval and setting of conditions for use of plant protection products. The scheme is detailed in the book and an example of the risk assessment of Metosulam is provided. Details of the RIVM USES scheme for agricultural pesticides are also provided.
6.3 Genetically Modified Organisms
A risk assessment is required by EC Directive 90/220/EEC on 'the deliberate release into the environment of genetically modified organisms' before consent is approved on the marketing or deliberate release of Genetically Modified Organisms (GMOs). Most EU States have developed methodologies for the risk assessment of GMOs and have organisations conducting research to refine the methodologies used to protect man and the environment (details can be found on the Belgian Biosafety Server on the Internet).

Pete Addis, Environmental images
|
There has been much criticism of the current methodologies used for the assessment of GMOs, particularly of the EC Directive guidelines. Concerns include the fact that our current knowledge does not provide us with the means to predict the ecological long-term effects of releasing organisms into the environment (von Schomberg, 1996) and the inconsistency of definitions between EU states leading to different criteria for approval (Levidow et al., 1996). Work is currently underway by the European Commission to address this problem of a lack of harmonisation (example).
|
|
Disharmony between GMO risk assessment approaches
Because of several difficulties discovered in the implementation of the 1990 Directive on Deliberate Releases of Genetically Modified Organisms into the Environment, the Directive has been under review. The Directive specifies the data which member states must obtain form companies and assess to decide whether to approve experimental or commercial releases. However, it appears that they have approached assessments with different objectives in mind, resulting in a "lack of harmonisation". Guidelines are currently being drawn up by the European Commission in an attempt to address this problem (ENDS Report, 1996).
|
At an international level, UNEP has been influential in the establishment of an international agreement on biodiversity, the Convention on Biological Diversity, signed by over 150 nations. This body is now working towards the creation of a Biosafety Protocol to be enacted in 1999. The work includes the evaluation of the existing policies and guidelines of the OECD, the FAO and the International Technical Guidance on Safety in Biotechnology being finalised by UNEP.
In the UK Guidance Document, risk assessment consists of seven steps (risk management is also integrated within the procedure). The book describes the steps and provides an example of the results of an assessment for the genetic modification of oil seed rape.
References for the chapter
Many of the sections in the Information Sources are relevant to this chapter but particularly:
Databases (chemicals)
Software Models (exposure)
Publications (Books)
|



Document Actions
Share with others